FEATURESFeatures of our medical products
With our medical products we contribute to improving the quality of life for patients. We offer a pressure infusion device, which combines a PCA device with a flow controller developed by using our unique technology and a flow control tube which takes advantage of our expertise accumulated through the manufacturing of nibs. We also offer a lineup of guide wires in pursuit of safety and functionality which have been developed for various medical settings.
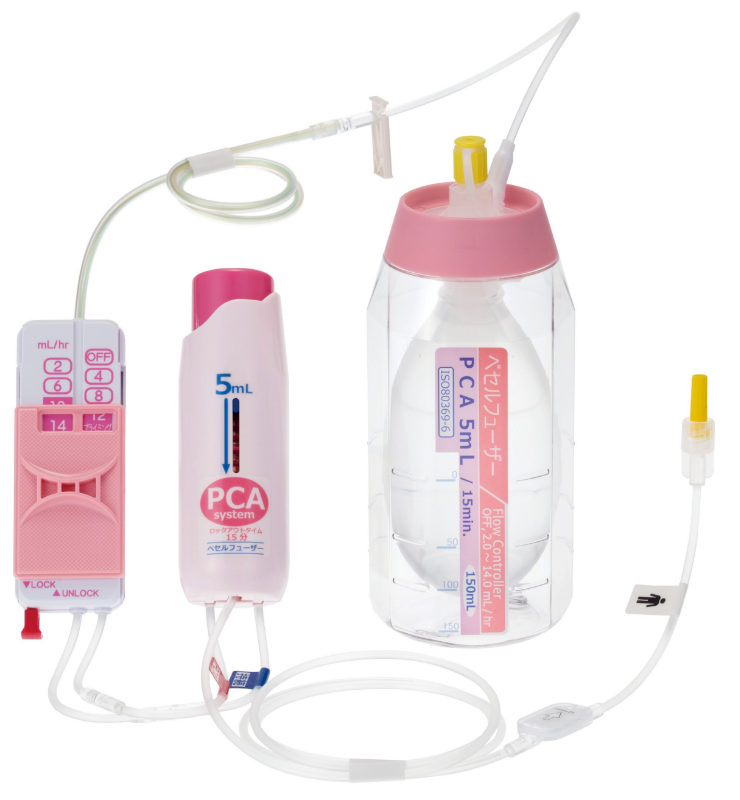
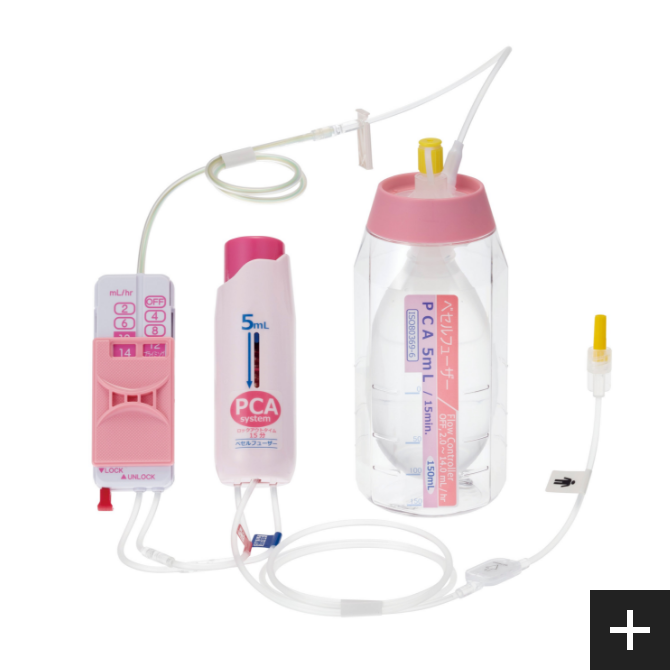
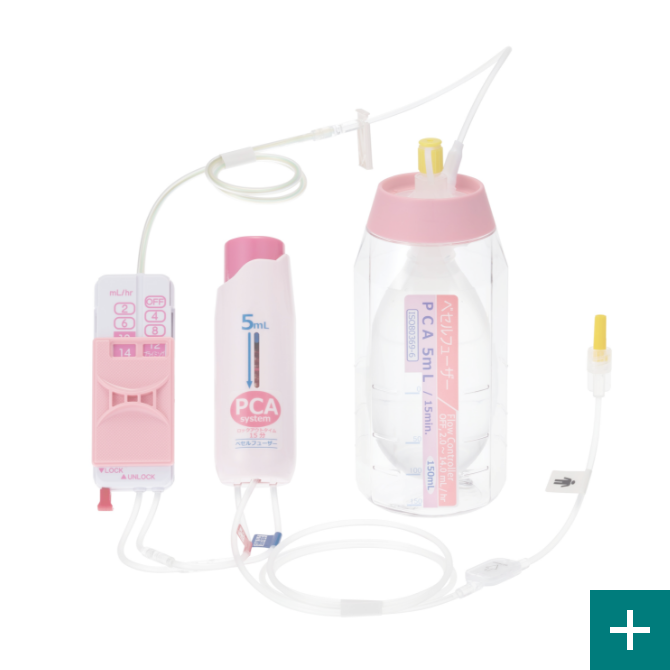
Achievement of design and development technologyVESSEL FUSER
Base born from our flow control technology VESSEL FUSER (pressurized medicament injector), by using a combination of control tube and the flow rate switching device (Flow Controller), can be switched at 12 stages. This per hour flow rate, can be controlled from 0.5mL. This is our own developed technology.
Flow controller, we do everything from product design* from manufacturing equipment to the assembly of finished products for many of the structures, such as flow control tube.
* There are some parts manufactured outside.

-
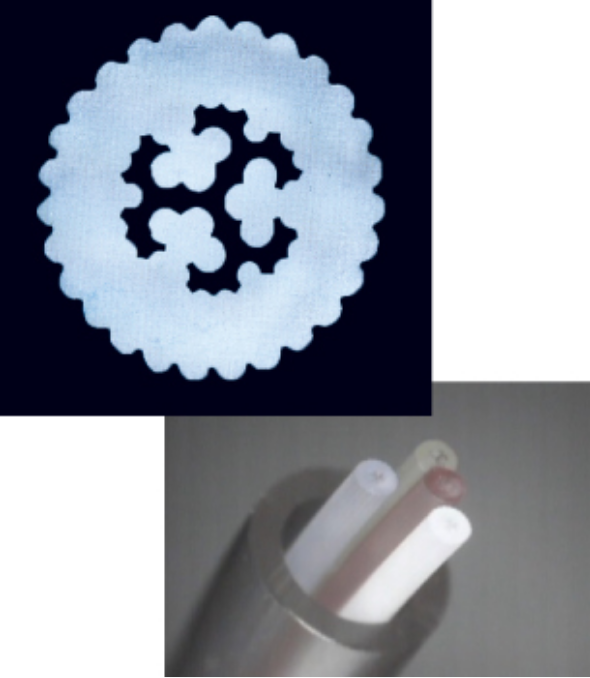
Micro flow rate control core
Unique control using pen nib fabrication technology controls the flow rate of a slight amount of chemical solution by a special circular cavity shape. It is also characterized resistance to kink (bending, worn-out) by this shape and coating.
-
Flow controller
The flow controller enables flow switching at multiple stages of up to 12 stages by combining four control cores with different flow rates and switching sliders.

-
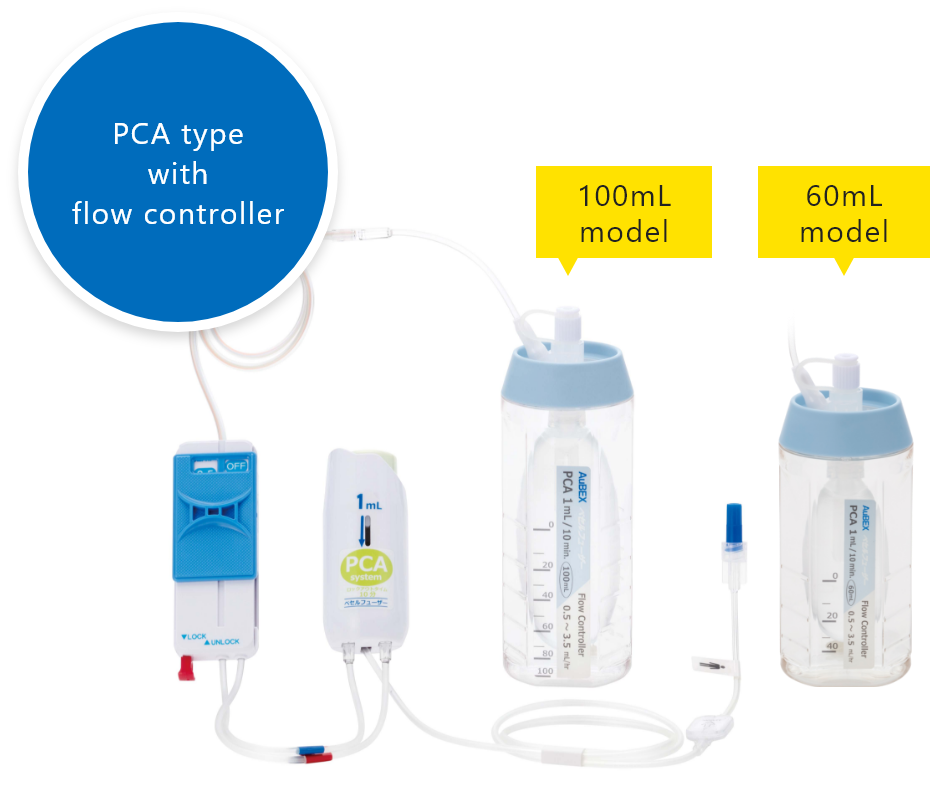
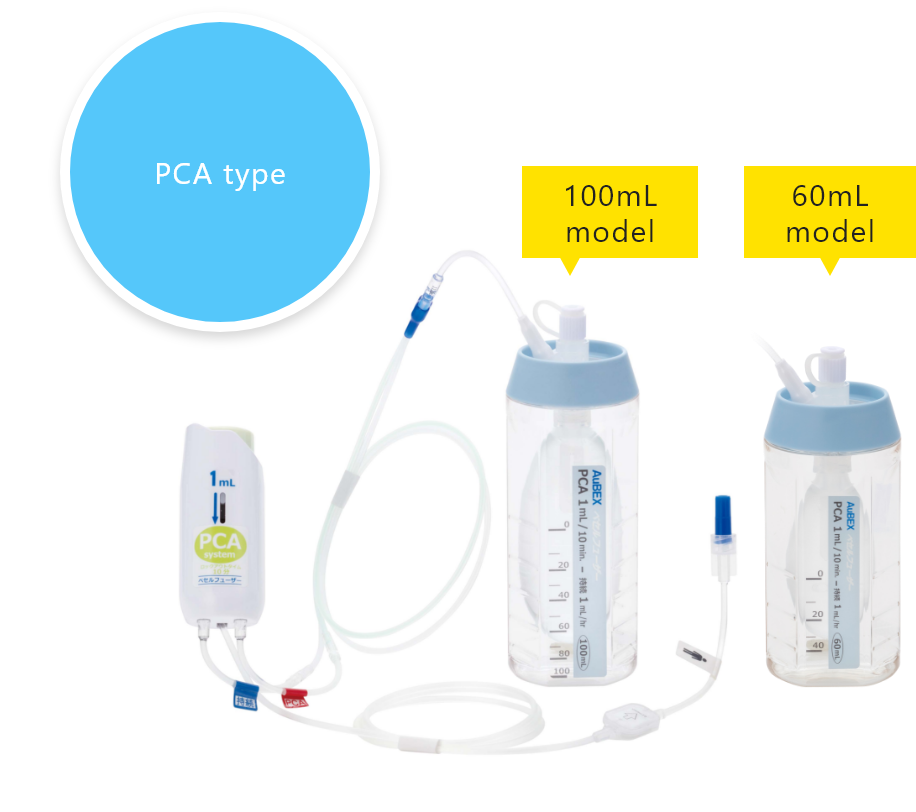
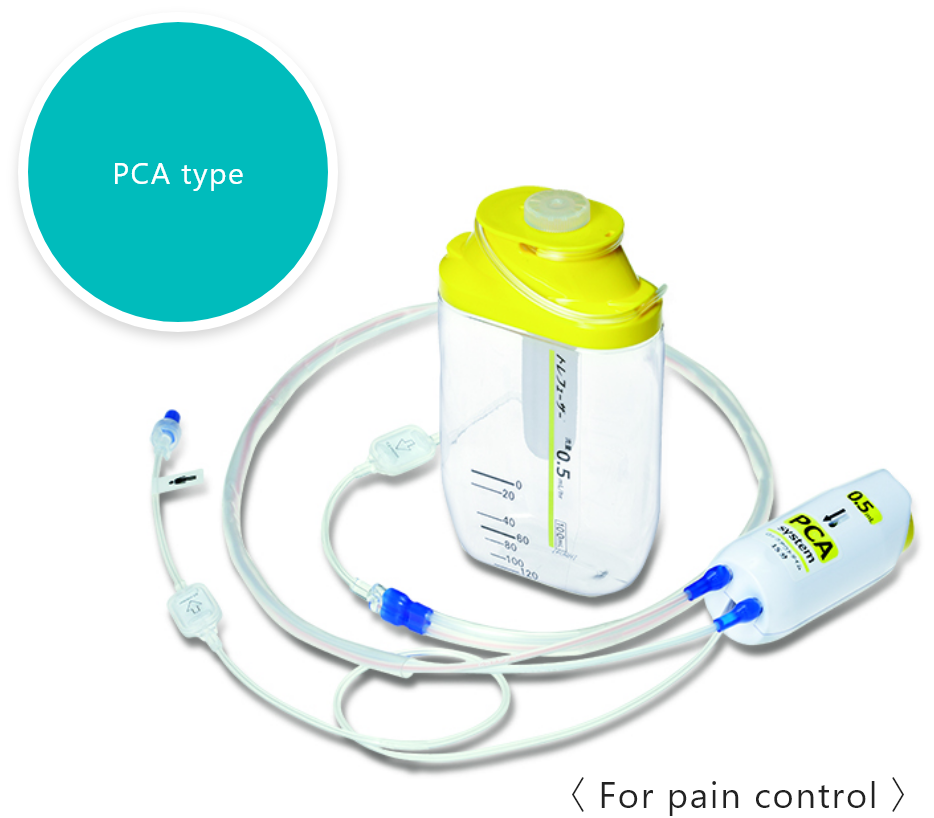
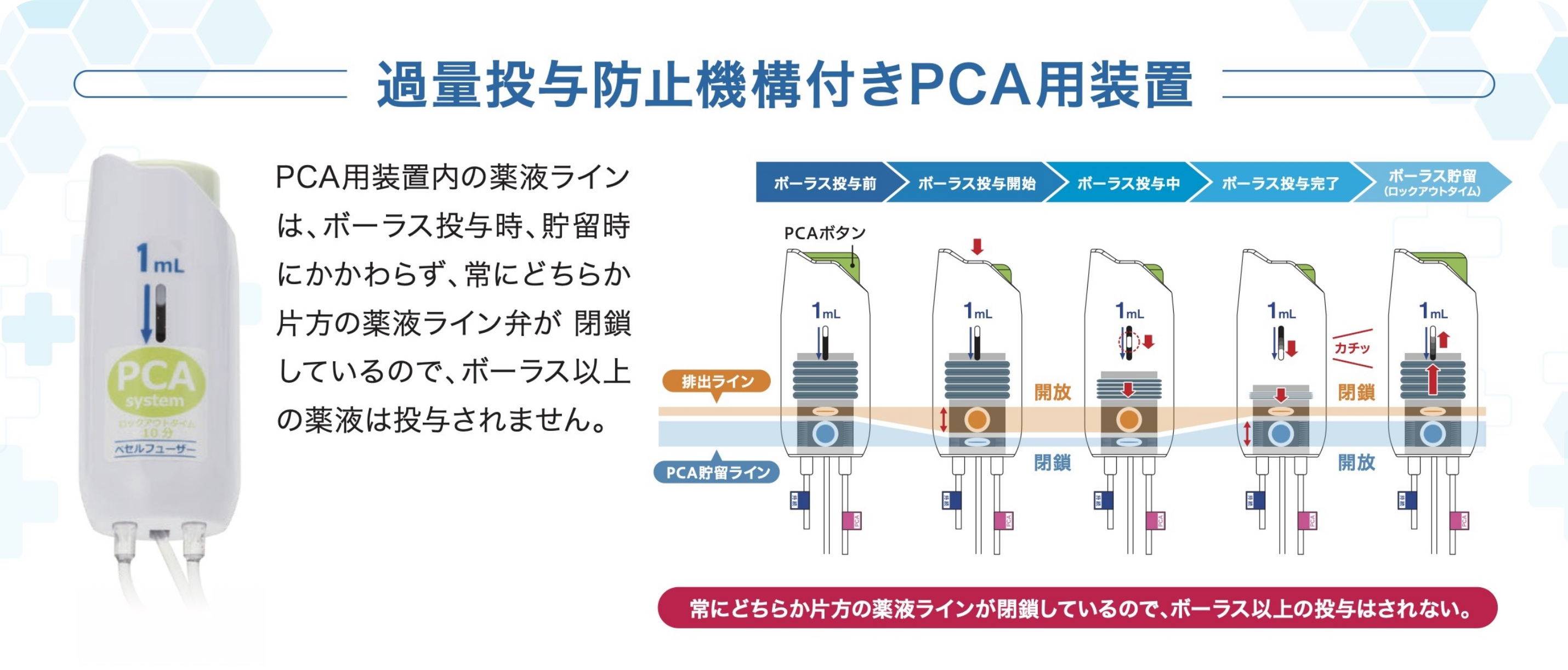
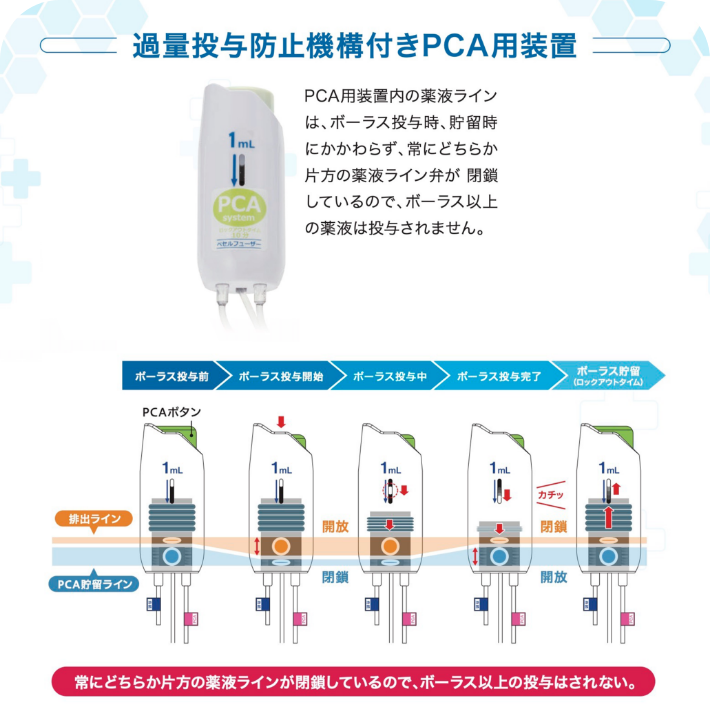
PCA device
A PCA* device enables patients to administer additional drug solutions, such as pain-killing drugs, on their own. It allows for a temporary increase in the amount of pain-killing drugs and other drug solutions supplied from the drug solution reservoir.
*PCA stands for "patient controlled analgesia".
LINE UPProduct lineup
-
For obstetric anesthesia
-
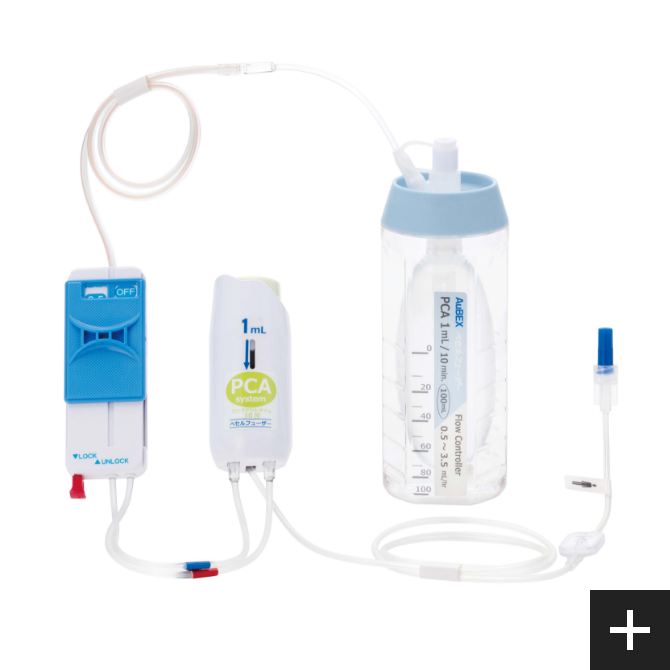
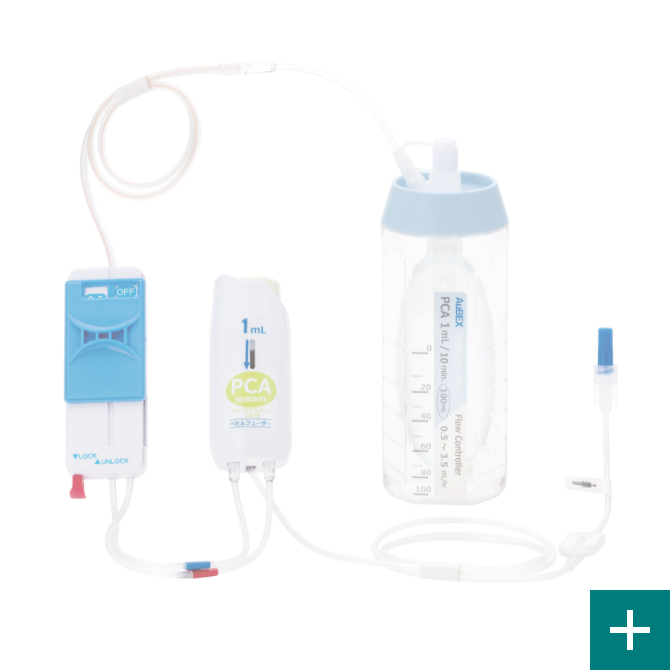
For post-surgical pain treatment
-


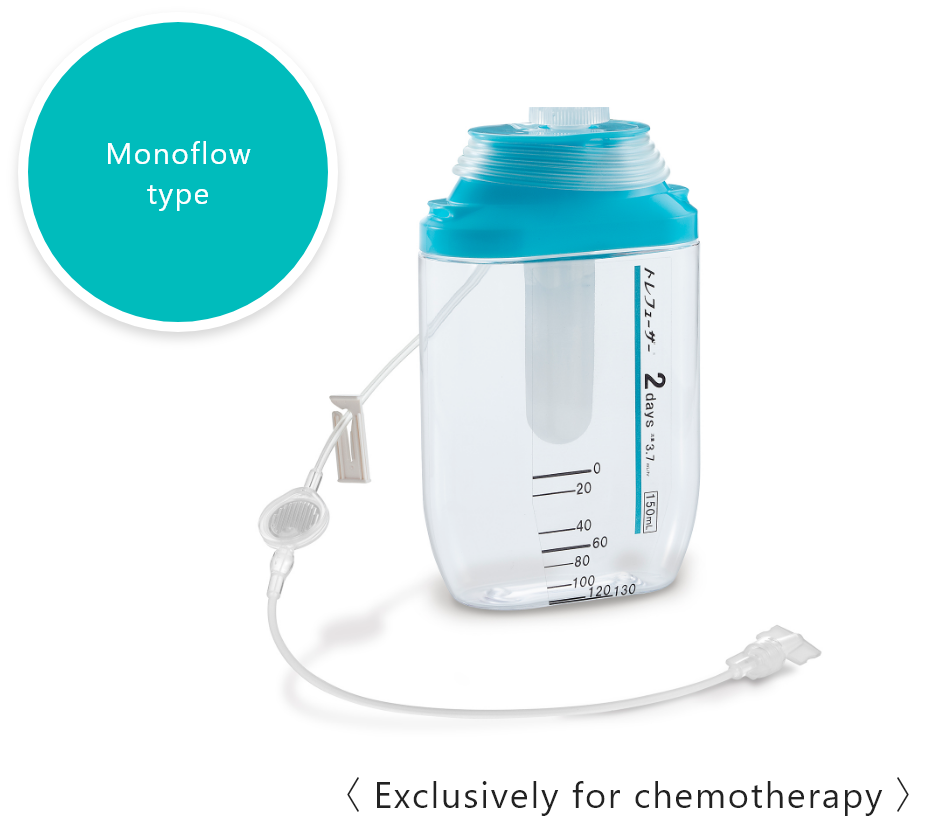
Medication therapy
-


Guidewire for contrast radiography
-
Medical devices and other products
Precautions and other information
(formerly known as package inserts)
Available by searching on the Pharmaceuticals and Medical Devices Agency (PMDA) website
For precautions and other information, ask the manufacturers and distributors of individual products.
Medical device data search website